Introduction
Aldeyra Therapeutics (NASDAQ:ALDX) is a biotech firm focused on creating therapies for immune-mediated diseases. Their groundbreaking RASP (reactive aldehyde species) pharmaceutical platform targets pro-inflammatory mediators contributing to inflammation-based diseases. Their product pipeline features several RASP modulators, including the orally administered ADX-629. They also have products under FDA review, such as reproxalap for dry eye disease and ADX-2191 for a rare cancer type, primary vitreoretinal lymphoma, and two inflammatory retinal diseases.
Aldeyra pipeline (Aldeyra 10-K)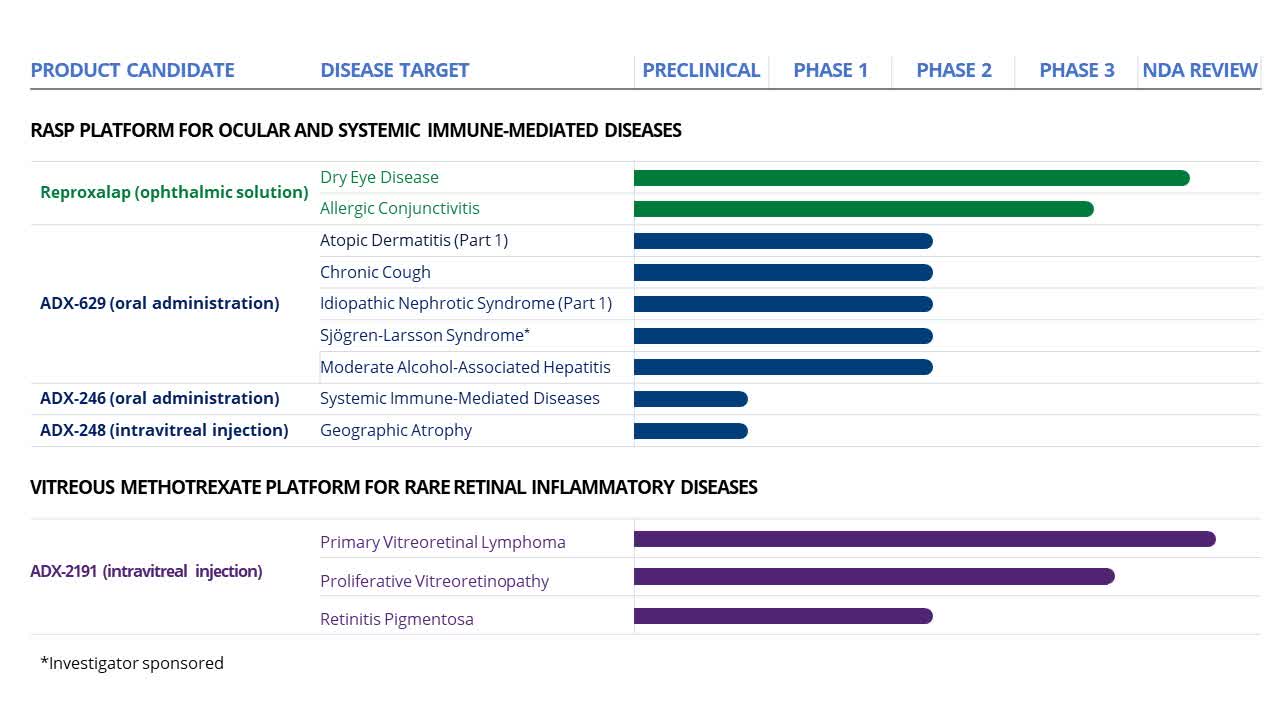
Recent Developments: Aldeyra recently achieved two significant milestones. Firstly, the company joined the Russell 2000 and Russell 3000 indexes on June 23, 2023. Additionally, Aldeyra announced positive phase 2 trial results for ADX-2191, its candidate for retinitis pigmentosa. The therapy demonstrated notable improvements in retinal function, with no reported safety issues. Aldeyra now intends to engage in discussions with regulators regarding a phase 2/3 trial for further evaluation.
This article primarily discusses Aldeyra’s reproxalap, a potential treatment for dry eye disease and allergic conjunctivitis. In my opinion, this stands as their most noteworthy and valuable asset.
Q1 2023 Earnings
As of March 31, 2023, Aldeyra Therapeutics reported $165 million in cash and cash equivalents. The company anticipates that this will fund operations, including the potential commercialization and launch of reproxalap and ADX-2191, along with the ongoing development of its product candidates until the second half of 2024. For Q1 2023, the company incurred a net loss of $15.6 million or $0.27 per share, compared to a net loss of $16.8 million or $0.29 per share in Q1 2022. Research and development expenses decreased by $1 million to $11.2 million, largely due to reduced external clinical development costs. However, general and administrative expenses increased by $1.4 million to $5.6 million, mainly because of higher personnel and legal costs. Total operating expenses for Q1 2023 were $16.8 million, slightly higher than the $16.5 million reported in Q1 2022.
Reproxalap: Transforming Eye Disease Treatment, Awaits FDA Nod
Reproxalap, a groundbreaking topical RASP modulator, is under FDA review for treating dry eye disease. This potential treatment, which has demonstrated efficacy and safety in numerous clinical trials, awaits a PDUFA decision in November 2023. Notably, reproxalap offers rapid relief from symptoms within minutes of application, while maintaining effectiveness for up to 12 weeks. This dual-action performance could significantly transform the management of dry eye disease.
The application to the FDA includes data from “over 2,300 patients”, highlighting reproxalap’s safety. Although minor, transient irritation at the site of application was reported, there have been no substantial safety concerns.
In addition to its potential application for dry eye disease, reproxalap has shown promise as a treatment for allergic conjunctivitis. This condition, frequently co-occurring with dry eye disease, affects an estimated 66 million people in the US, per Aldeyra. Reproxalap has shown a potent ability to alleviate key symptoms such as ocular itching and redness.
Recently, Aldeyra reported positive outcomes from the Phase 3 INVIGORATE-2 clinical trial of reproxalap in patients with allergic conjunctivitis. The trial reached statistical significance for both its primary and all secondary endpoints, displaying a significant reduction in ocular itching at all specified timepoints. Similarly, it exhibited statistically significant improvements in key secondary outcomes such as ocular redness and tearing. These findings align with prior Phase 2 and Phase 3 trials, supporting reproxalap’s potential as a treatment for inflammatory conditions of the ocular surface.
Should reproxalap gain FDA approval, it could penetrate a considerable market due to the prevalence of dry eye disease and allergic conjunctivitis. The drug’s unique attributes, robust clinical data, and minimal safety concerns suggest a promising market potential. Moreover, the opportunity to treat allergic conjunctivitis could broaden its market appeal even further.
My Analysis & Recommendation
In conclusion, Aldeyra Therapeutics, with its diverse pipeline and innovative RASP modulator platform, appears to be poised to make a significant impact in the sector of immune-mediated diseases, particularly in treating dry eye disease and allergic conjunctivitis. Reproxalap, currently awaiting FDA approval, has demonstrated compelling safety and efficacy in clinical trials, setting it apart from existing treatments. Its rapid symptom relief and sustained effectiveness could potentially disrupt the treatment paradigm for these prevalent conditions.
Given the prevalence of dry eye disease and allergic conjunctivitis affecting millions of individuals in the US alone, the market opportunity for reproxalap is vast. If it receives FDA approval, its unique characteristics, strong clinical data, and its dual functionality for treating both conditions could provide Aldeyra with a strong competitive edge.
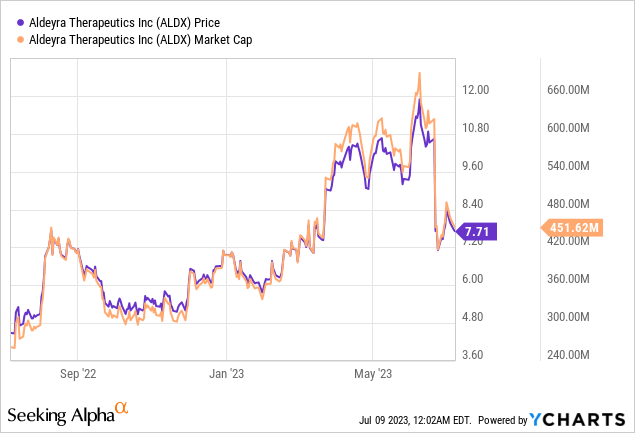
Valued at a market cap of $450 million, Aldeyra Therapeutics seems to present an attractive investment opportunity. Its strong cash position, expected to fund operations into the second half of 2024, mitigates near-term financial risk. Although the company reported a net loss in Q1 2023, the lower R&D expenses indicate effective cost management and the increase in G&A expenses can be viewed as investment towards future growth.
However, the inherent risks associated with biotech investments should be considered. Regulatory approval is not guaranteed and a negative decision could adversely affect the company’s prospects. Therefore, potential investors should weigh these risks against the substantial market opportunity and the promising clinical data.
Overall, given these considerations, Aldeyra Therapeutics could be seen as a ‘Buy’ opportunity for investors with a long-term perspective and a tolerance for the risk profile typical to the biotech sector. The anticipated PDUFA decision in November 2023 serves as a key potential catalyst that investors should closely monitor. A positive outcome could significantly enhance Aldeyra’s market position and shareholder value.
Risks to Thesis
When the facts change, I change my mind.
While I maintain a positive outlook on Aldeyra Therapeutics, there are several risks to my bullish thesis that I must consider:
- Regulatory Risks: Despite the promising results observed in clinical trials, there is inherent uncertainty surrounding the approval of reproxalap by regulatory authorities. The non-approval of reproxalap by the FDA could have a significant impact on the company’s future prospects and the value of its stocks. Apart from reproxalap, Aldeyra has other candidate drugs that are also exposed to regulatory risks. For instance, in June, the marketing application for ADX-2191, intended for primary vitreoretinal lymphoma, was denied, leading to a drastic 20% decline in the value of Aldeyra’s stock.
- Market Acceptance: If reproxalap is approved, there is still the risk of whether it will be accepted by patients and medical professionals. Moreover, the drug’s market penetration will depend on how it stacks up against existing treatments in terms of effectiveness, cost, and side effects.
- Financial Risks: While Aldeyra appears to be in a solid financial position at the moment, continued net losses could affect the company’s ability to sustain operations in the long term. If additional capital is required, it could dilute existing shareholders’ equity.
- Competition: The biotech sector is highly competitive. Other companies might be developing similar or more effective therapies for the same conditions. If a competitor’s drug gets to the market first or is deemed superior, it could diminish the market potential for reproxalap.
- Legal and Patent Risks: The biotech sector often faces legal challenges related to patents. If Aldeyra’s patents are challenged, it could lead to costly legal battles and potential loss of exclusivity for their drugs.
Read the full article here





