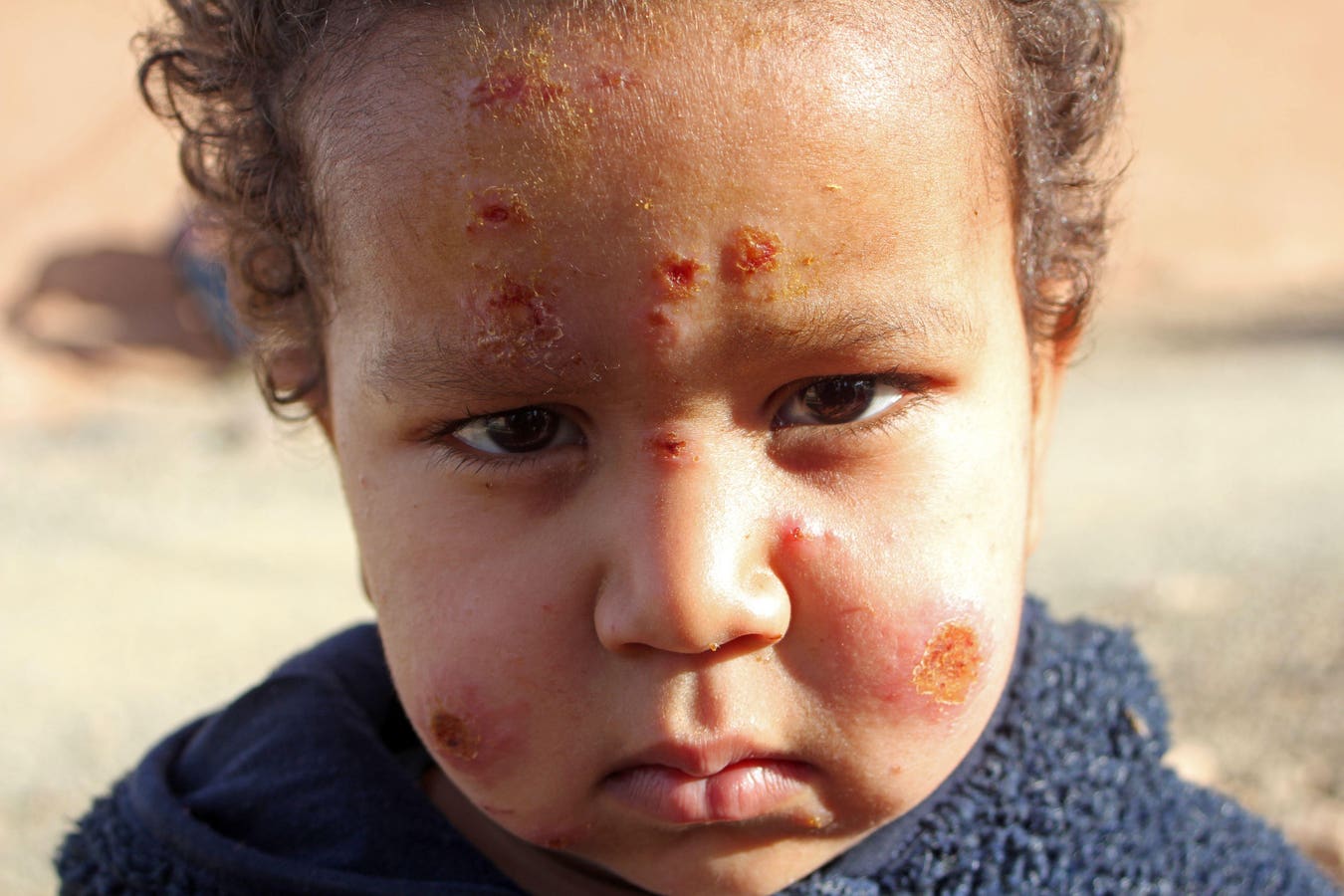
A disfiguring parasitic infection, common in the tropics, has now found a home in Texas and Oklahoma and is expanding its range here in the U.S. The parasite, Leishmania mexicana, is transmitted by tiny sand flies, which are now found in Maryland, Delaware, New Jersey, and Ohio.
In a recent report from the Centers for Disease Control and Prevention, researchers examined 2,100 skin samples. Of these, 1,222 showed the presence of leishmania, and, as expected, almost all the patients had traveled internationally to endemic areas (where the organism commonly occurs). However, 86 patients had no travel history, meaning they acquired the infection in the U.S.
There are different strains of leishmania, and they cause different types of disease. The L mexicana found in Texas and Oklahoma causes the mildest form, cutaneous leishmania, affecting only the skin. It generally requires no treatment. L brasiliensis causes mucocutaneous leishmania, which I saw while studying in Peru. At that time, it was treated with a nasty antimonial antibiotic called Pentostam with many side effects.
The most severe form of leishmania occurs in the Middle East, Asia, and northern Africa. L donovani species causes this systemic illness called kala-azar. It is visceral—infecting organs throughout the body, including the liver and spleen—and often causes death.
The scientists also did special genetic studies of the L mexicana in Texas and found two distinctive genotypes. They called one type “CCC” and found it in 94% of the non-travelers. While the “American strain” was found in samples as long as 18 years ago, Dr. David Freedman, Professor Emeritus of Infectious Disease at UAB at Birmingham, said that Leishmania has probably “been there forever.” He was aware of its presence in Texas in the 1980s. It was “under the CDC’s radar” because the skin lesions usually resolve without any treatment and because it is not a reportable disease.
Spread:
Leishmania is spread through bites of sand flies. Their normal hosts in the US are wood rats (not common Norway or city rats). Anne Straily (CDC Veterinary Medical Officer) said via email, “There is some debate about whether other wildlife species, like armadillos or opossums, could also serve as reservoir hosts.” In some cases, infected humans can transmit the parasite back to sand flies.
For visceral leishmania, the most serious form, dogs are the main reservoir. Per Mary Kamb (CDC medical epidemiologist) and Vitaliano Cama (CDC microbiologist), L. infantum (the species that causes the visceral form of disease) “is already present in certain dogs in the U.S., primarily in hunting hounds.”
Many dogs are being imported to the U.S. as rescues from SE Asia, and posing a potential risk here. “No sand flies have been found infected with L. infantum in the U.S., and no human cases of locally acquired visceral leishmaniasis caused by L. infantum have been identified, even among people in contact with infected dogs,” Kamb and Cama said via an email interview.
An interdisciplinary working group, including the CDC and public health veterinarians, has developed a new “operational risk assessment tool” to help vets recognize and screen dogs that pose the greatest threat. This tool was presented at the recent American Society of Tropical Medicine and Hygiene meeting.
Also of concern is that one-fifth of the 2.7 million soldiers deployed to Iraq or Afghanistan have evidence of asymptomatic visceral leishmania, including parasites in their blood, up to ten years after returning. They could potentially transmit their infection through insect bites, pregnancy, transfusions, or IV drug use. A 27-month-old child from North Dakota, who had never left the state, was found to have a skin infection with L donovani, which causes the visceral form of disease. It’s unclear exactly how transmission occurred; his mother was from Nepal, but there is no report of having tested her.
Prevention:
Sand flies are much smaller than mosquitoes, so unless the mesh is sized accordingly, bed nets are less effective in preventing bites. Data on results is mixed, but there is some benefit to insecticide-treated nets. These are usually used in rooms that are also sprayed with insecticides.
For personal use, DEET and permethrin are effective preventatives.
Challenges:
The initial hurdle is getting physicians to diagnose leishmania. Many will not likely have ever seen it nor suspect it. Even on biopsy, it may be missed as the organisms are so tiny, and a special Giemsa stain is required. You won’t know the species from appearance, Freedman explained. Diagnosis is generally done by molecular (PCR) testing at the CDC. Testing is now commercially available at the University of Washington for anyone or at Walter Reed Army Institute of Research (WRAIR) (military only).
The other major problem is that there are no reporting requirements except in Texas, or to the CDC. Without a uniform reporting to the CDC, we have no chance of tracking how frequently and where it occurs.
The magnitude of the role of climate change in the spread of leishmania is as yet unknown—this is another reason to have careful tracking.
Insecticide resistance is a concern. The Covid pandemic reduced funding for leishmania programs and surveillance and resulted in less spraying.
We also need to watch that the visceral type of leishmania does not become endemic in the US, becoming established by sand flies feeding on infected dogs. This hasn’t happened yet (that we know of) but requires ongoing monitoring.
For many unusual infections, it’s important that physicians take a careful exposure and travel history and have a high index of suspicion. It’s also important that physicians learn to recognize cutaneous leishmaniasis and know that it can occur in people with no history of international travel.
Read the full article here