This story is part of a series on the current progression in Regenerative Medicine. This piece is part of a series dedicated to the eye and improvements in restoring vision. This piece also marks part three in a small series on diabetic retinopathy.
In 1999, I defined regenerative medicine as the collection of interventions that restore to normal function tissues and organs that have been damaged by disease, injured by trauma, or worn by time. I include a full spectrum of chemical, gene, and protein-based medicines, cell-based therapies, and biomechanical interventions that achieve that goal.
Diabetic retinopathy is a progressive eye disease that affects individuals diagnosed with diabetes. Diabetic retinopathy happens when elevated blood sugar levels cause damage to the blood vessels in the retina. The retina plays a crucial role in transmitting visual images to the brain. As the condition progresses, these damaged blood vessels can leak or become blocked, leading to vision problems and potential blindness. In recent years, significant efforts have explored the potential of NOX4 inhibition as a novel therapeutic approach for diabetic retinopathy.
A Very Small Explanation of Diabetic Retinopathy
Diabetic retinopathy is a complication of diabetes that leads to vision impairment and blindness. The disease’s molecular basis is intricate and involves several mechanisms that respond to high blood sugar levels, including oxidative stress, inflammation, and vascular dysfunction. One of the critical molecular players in diabetic retinopathy is a protein called NOX4, a member of the NADPH oxidase family of enzymes that generate reactive oxygen species (ROS) in cells.
NOX4 is expressed in retinal cells, including vascular cells, affected by diabetic retinopathy. Studies have shown that NOX4 promotes oxidative stress and inflammation in the retina, leading to damage to the blood-retinal barrier (BRB) and the development of early and advanced stages of diabetic retinopathy. NOX4 also activates several signaling pathways that contribute to the disease’s pathogenesis, including the AGE-RAGE pathway, the protein kinase C (PKC) pathway, and the vascular endothelial growth factor (VEGF) pathway.
Therefore, NOX4 has emerged as a promising therapeutic target for treating diabetic retinopathy. Several preclinical studies, such as one by the University of Crete, have shown that inhibiting NOX4 activity can reduce oxidative stress, inflammation, and vascular dysfunction in the retina, preventing the development and progression of diabetic retinopathy.
Inhibiting NOX4 in the Retina
Among the numerous NOX4 inhibitors developed for this purpose, an inhibitor called GLX7013114 has emerged as an up-and-coming candidate. The previously mentioned study by the University of Crete delved deeper into the effects of this NOX4 inhibitor in vivo using two experimental models of diabetic retinopathy.
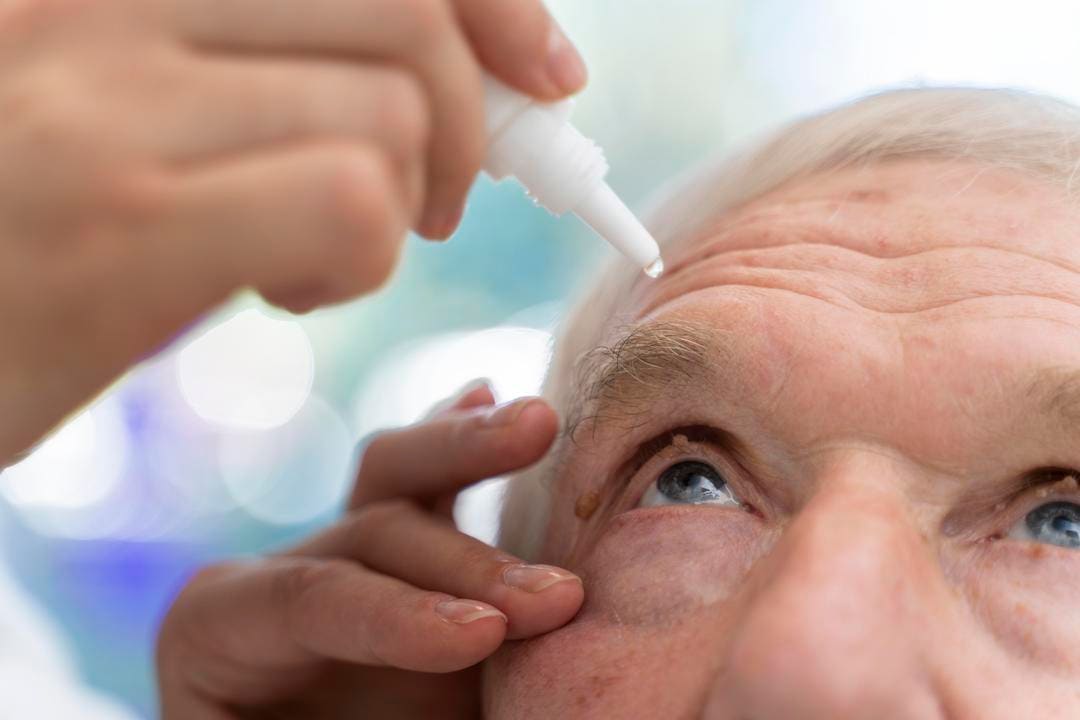
The study found that when administered topically as eye drops, GLX7013114 exhibited considerable beneficial effects in reducing oxidative stress, neurodegeneration, and neuroinflammation in the diabetic retina. Additionally, the NOX4 inhibitor mitigated the diabetes-induced increase in vascular endothelial growth factor (VEGF) levels and proinflammatory cytokine levels in the retinal tissue. Furthermore, GLX7013114 exhibited neuroprotective, anti-inflammatory, and vasculoprotective properties, which can protect retinal ganglion cell function.
Another study published in Redox Biology also found similar results. The study highlights NOX4’s importance in controlling redox signaling in high glucose and regulating oxidative stress. The article also briefly discusses NOX4’s relationship with diabetic macroangiopathy and outlines potential areas for future research.
These encouraging findings highlight the exciting prospects of GLX7013114 as a potential therapeutic intervention for diabetic retinopathy. The continued research and development in this field hold promise for preventing and managing this visually impairing condition, ultimately improving the lives of individuals affected by diabetic retinopathy.
Other Therapeutic Agents
Researchers are exploring alternative therapeutic targets that can complement or replace NOX4-based treatments to counteract the effects of AGEs and develop more effective treatments for diabetic retinopathy. Among these targets are protein kinase C and advanced glycation end products.
Protein kinase C encompasses a group of enzymes that play a crucial role in orchestrating diverse cellular functions like cell growth, differentiation, and programmed cell death. These enzymes wield significant influence over fundamental cell processes, working together harmoniously to ensure proper cellular functioning. Multiple signals, such as hormones, growth factors, and stress, activate them. Once activated, they phosphorylate target proteins, modulating their activity and function. There are several isoforms of protein kinase C, each with a distinct expression pattern and localization within the cell.
Recent research has shown that protein kinase C plays a significant role in developing diabetic retinopathy and other diabetic microvascular complications. In diabetic retinopathy, protein kinase C is activated by high glucose levels, leading to increased vascular permeability, inflammation, and oxidative stress. This, in turn, contributes to the development of retinal edema, hemorrhages, and neovascularization. Protein kinase C has also been implicated in other microvascular complications of diabetes.
In the case of diabetic retinopathy, advanced glycation end products are believed to contribute to microvascular damage and inflammation in the retina. AGEs are compounds formed when proteins and sugars undergo glycation. They play a significant role in diabetic complications such as neuropathy, nephropathy, and retinopathy. This irreversible process creates cross-linked molecules resistant to the body’s natural turnover and removal mechanisms. AGEs contribute to microvascular damage, inflammation, and abnormal blood vessels in diabetic retinopathy.
Where Do We Go From Here?
Ongoing scientific research is expected to provide in-depth insights into the intricate molecular mechanisms involved in diabetic retinopathy, a common complication of diabetes. By understanding these mechanisms, researchers can develop more effective inhibitors that can help prevent or treat the condition. These advances in research offer exciting prospects for the potential treatment of diabetic retinopathy, which could improve the quality of life for millions of people living with this condition.
To learn more about the eye, read more stories at www.williamhaseltine.com
Read the full article here