What if CAR T cells, revolutionary cancer fighters normally manufactured in the lab, could be created inside the patient? This quest to simplify CAR T therapy has brought two biotech companies to a similar answer: viral injections. According to research presented at the 2023 American Society of Hematology conference, lentiviral injections could transform patient cells into CAR T cells without outside manipulation. If clinically translated, the concept promises to streamline current protocols and increase the therapy’s accessibility.
CAR T Therapy: An Imperfect Treatment
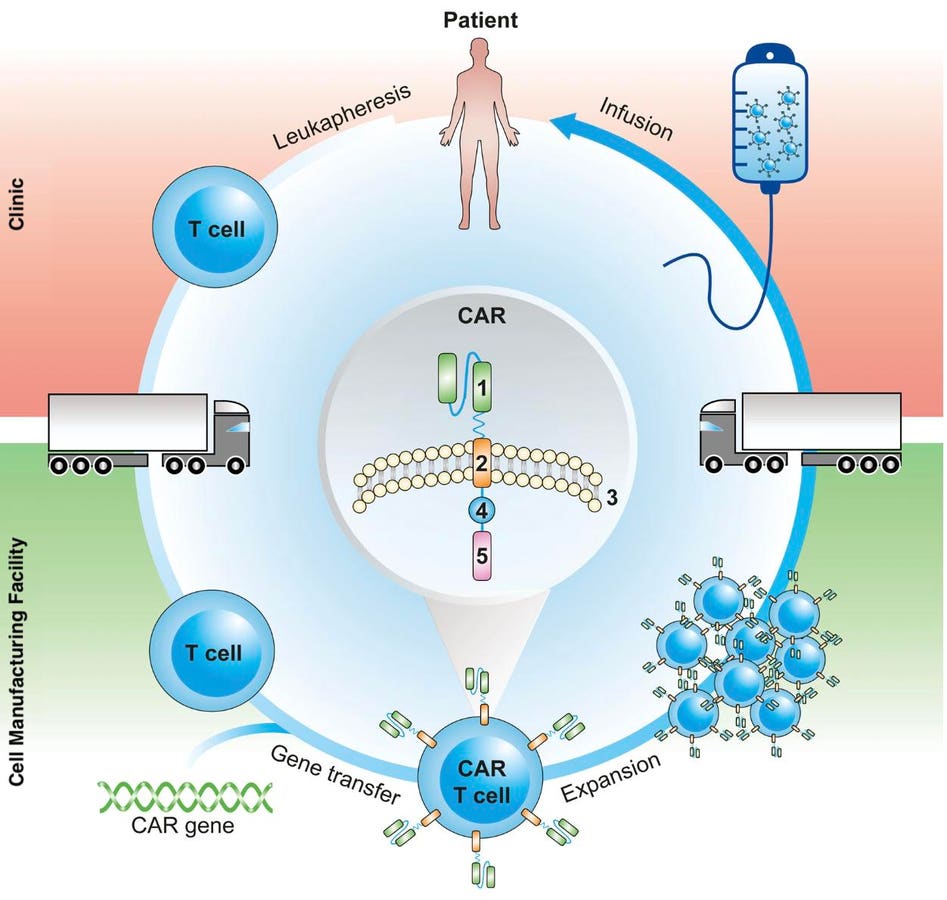
Chimeric Antigen Receptor T cell (CAR T) Therapy is a novel treatment that enhances the body’s natural ability to kill certain blood cancers. The process entails extracting immune cells called T cells from the patient. The cells are then sent to a lab, purified, bioengineered to express a synthetic receptor, and multiplied to great numbers. Finally, the patient undergoes lymphodepleting chemotherapy before receiving their CAR T cell infusion. The CAR receptor allows the T cells to detect a specific protein found on blood cancer cells and destroy them.
Although CAR T therapy can achieve durable responses for patients with relapsed/refractory leukemias, lymphomas and multiple myeloma, it remains inaccessible to most who need it. One contributing factor is the complex manufacturing. The cells are crafted in specialized facilities and tailored to each patient. The treatment requires more time, resources, and personnel to develop than ready-made medicines—all factors that drive up the cost.
Another potential limitation is the required lymphodepleting chemotherapy. The procedure increases a patient’s susceptibility to dangerous infections and prolonged periods of low blood cell counts. Opportunistic viruses in the body may also reactivate in light of this immunosuppression. For some, the associated risk is critical to consider before electing CAR T therapy.
Studies on viral vector-delivered, in vivo CAR T therapy seek to address these concerns. Let’s go into more detail.
Why Go In Vivo with CAR T Therapy?
To combat complications associated with standard CAR T cell production, some researchers place their hopes on an in vivo CAR T therapy. This concept aims to eliminate the complexity and high costs that plague externally manufactured cell therapies by creating the CAR T cells within the patient instead of in a lab. This alternative skips the T cell extraction, purification and modification typically required. In vivo CAR T therapy promises to be scalable, which would make the treatment easier and more accessible to produce. Additionally, lymphodepleting chemotherapy may not be needed to prime the patient for the therapy.
A preclinical trial of mice with heart damage demonstrated that in vivo CAR T cells can be made with a lipid nanoparticle injection. This method mirrors the gene delivery featured in COVID-19 mRNA vaccines. The nanoparticles act as a vehicle, delivering the desired chimeric receptor genes to the patient’s T cells. The T cells temporarily express the synthetic receptor and significantly reduce heart damage in mice.
Recently presented research suggests that viral injections could accomplish similar feats. Viral vectors could be designed to treat cancers via injection, much like a vaccine for an immunocompetent person.
In Vivo CAR T Therapy with Viral Injections
During the last annual ASH conference, two companies presented their latest work on in vivo CAR T therapy. Although the products are distinct, the two share similar features, such as the use of lentiviral injections to deliver CAR genes to the T cells inside a living animal.
Lentiviral vectors are commonly used to deliver genes in cell therapies, and CAR T therapy is no exception. This vector is made from nonreplicating forms of the lentivirus HIV. Researchers take advantage of HIV’s propensity to infect T cells by inserting synthetic genes into the virus. The virus then delivers the gene package to the desired cell. This strategy integrates CAR genes into a patients’ T cells in the lab.
Can viral vectors deliver results in vivo, too? Umoja Biopharma presented groundbreaking preclinical data at the conference suggesting that lentiviral vectors can generate CAR T cells in vivo. Their CAR T therapy VivoVec™ relies on lentiviral particles that carry multi-domain fusion (MDF) proteins on their surface. The protein contains T cell activating and costimulatory domains alongside a CAR transgene against antigen CD20, a protein commonly found on the surface of cancerous blood cells.
In humanized mice models of cancer, a single shot of their viral particles into the lymph nodes leads to the efficient generation of CAR T cells without needing lymphodepletion. The CAR T cells are able to kill tumor cells, secrete cytokines and proliferate. Nonhuman primates that receive the viral injection demonstrate B cell aplasia, a sign of successful CAR T cell killing. The injection is well-tolerated and forms CAR T cell memory populations in the body.
Interius BioTherapeutics also announced their own in vivo CAR T therapy called INT2104. Here, the viral particle carries an anti-CD20 CAR gene; the particle’s surface is decorated with fusogens, proteins that promote the pH-dependent merging of two adjacent cells, and binder proteins that selectively target T cells.
Humanized mice with blood cancer successfully formed CAR T cells and demonstrated B cell depletion within seven days of receiving the viral injection into their tail vein. All treated mice experience complete tumor ablation and protection against tumor reformation. Macaques that receive the viral injection achieve at least a 75% reduction in circulating B cells. One macaque demonstrated a complete and prolonged B cell depletion; in this animal, CAR genes were detected in the peripheral blood and bone marrow.
Future Implications
In vivo CAR T therapy is gaining momentum as a faster, cheaper, and more accessible alternative to standard CAR T protocol. Delivering CAR genes directly to the patient without external cell manipulation may be critical to overcoming the therapy’s current limitations.
Viral vectors, already a staple within the industry, could be repurposed to transport the desired receptor genes to a patient’s T cells in vivo. The data presented at the ASH conference illustrates this method as a viable alternative to treating B cell malignancies. However, some caveats exist.
One potential caveat to using viral vectors is viral tropism, or a virus’s ability to infect certain cell types. While viral injections can be altered to CD7-bearing seek T cells and natural killer cells, as demonstrated here, it is important to note that HIV infects CD4+ helper T cells under normal conditions. This natural tropism to helper T cells could influence the treatment’s therapeutic effect in humans. Additionally, while in vivo CAR T therapy signifies a positive start to eliminating the arduous and potentially dangerous process of isolating, modifying and infusing cells into immunosuppressed cancer patients, this method will likely suffer similar limitations to traditional CAR T therapy: its failing application on solid tumors, and risk of causing secondary cancer in rare cases. This nascent frontier of CAR T therapy will be exciting to watch as more research unfolds.
Read the full article here