A new era of cancer care emerged in the 2010s with the creation of checkpoint inhibitor therapies. These treatments improved the immune system’s innate ability to kill tumors. Pembrolizumab, sold as Keytruda by Merck & Co., was one of the first inhibitors to hit the market. A trailblazer in its own right, similarly functioning checkpoint inhibitors arose in the wake of pembrolizumab’s approval in 2014. This article details how the therapy works, why it stands apart from other inhibitors and hints at potential future developments.
How Pembrolizumab Works
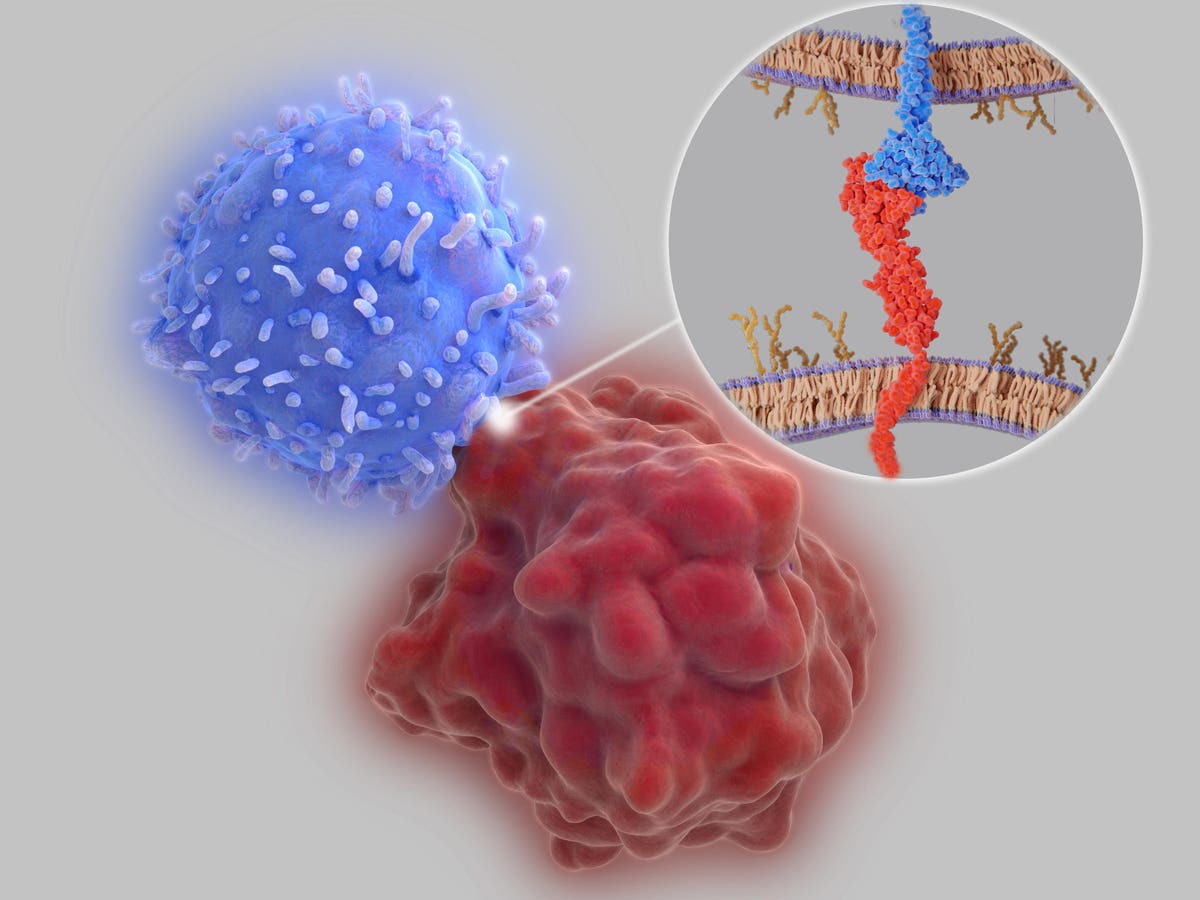
Pembrolizumab is an anticancer treatment that works by delivering antibodies into the bloodstream intravenously. The antibodies promote antitumor responses by binding to immune checkpoints, proteins found on the surface of immune cells.
Under normal circumstances, checkpoint proteins tamp down the immune system—both to promote immune tolerance, the process by which the body recognizes and avoids attacking its own healthy cells, and to prevent overstimulated immune responses. Tumors also hijack this system to grow freely. Cancer cells quiet immune cells that otherwise would attack by binding to these checkpoint proteins. Checkpoint inhibitors are prized for releasing these T cells which would normally be suppressed by the tumor, allowing them to launch an attack.
Pembrolizumab encourages T cells to fight by releasing their restraints. The therapy targets a specific checkpoint protein found primarily on T cells known as PD-1, or programmed death cell protein 1. By blocking access to the PD-1 checkpoint, other cells can no longer use that protein to stifle T cell activity. The T cell can then activate and retaliate appropriately.
When is Pembrolizumab Used?
Pembrolizumab can be a potent anti-cancer therapy. It is normally used for advanced cancers that have spread, have relapsed from previous treatments or cannot be removed with surgery alone. Patients receive an infusion every 3 to 6 weeks for up to two years.
How the immunotherapy is administered will vary depending on the disease. For some cancers, doctors can offer pembrolizumab as a first-line treatment. This is the case for qualifying patients with non-small cell lung cancer, throat cancer, gastric cancer or kidney cancer. It can also be given before or after surgery to prevent cancer reoccurrence. Other times, patients must exhaust other treatment alternatives before electing pembrolizumab.
Adding to the complexity, pembrolizumab can be administered alone or alongside other cancer treatments. It is often paired with chemotherapy, radiation and anti-cancer therapies; it is not, however, commonly combined with other checkpoint inhibitors.
The treatment was initially cleared to treat people with advanced melanoma. Today, it elicits antitumor responses in around 18 different types of cancers, including certain blood cancers and various solid tumors. This compatibility can likely be attributed to its broad mechanism of action, as many tumors exploit PD-1 checkpoints to suppress the immune system.
Note, however, that the drug’s efficacy is not uniform. Research suggests that pembrolizumab demonstrates improved efficacy for people with the following cancers:
- Melanoma
- Non-small Cell Lung Cancer
- Head and Neck Squamous Cell Carcinoma
- Classic Hodgkin Lymphoma
- Solid tumors with certain gene mutations
This inhibitor can clinically benefit other patients as well, albeit to a lesser extent; kidney, bladder, liver, colorectal and breast cancers reside within this list.
The First Approved PD-1 Checkpoint Inhibitor
Of the several checkpoint inhibitors on the market, five of these drugs target PD-1 checkpoint proteins, including pembrolizumab. How does pembrolizumab differ from these other inhibitors?
Pembrolizumab was the first anti-PD-1 therapy to receive federal approval in 2014. Actually, the first-ever approved inhibitor was ipilimumab which, previously described, acts upon a different checkpoint molecule called CTLA-4. All other PD-1 targeting inhibitors arrived after pembrolizumab.
Another divergence is how the immunotherapy is made. Pembrolizumab relies on humanized antibodies. In comparison, its PD-1 competitors use fully human antibodies.
Lastly, pembrolizumab is indicated to treat at least 18 types of cancers. It is used in more combinations against more tumors than ipilimumab or any other federally approved checkpoint inhibitor.
Diagnostic Test
A diagnostic test was developed to help identify which patients would respond best to pembrolizumab. To begin the test, a tissue sample is removed from the tumor. Pathologists then analyze the sample in the lab to see if the tumor cells express a receptor called PD-L1 (programmed death ligand 1) and at what concentration. Tumors that highly express this protein interact more readily with PD-1 checkpoints on T cells and therefore should be more responsive to pembrolizumab’s anti-PD-1 mechanism. The test is available to patients with advanced Head and Neck Squamous Cell Carcinoma (HNSCC), or people with certain breast, cervical, gastric or non–small cell lung cancers.
Possible Adverse Effects
Due to its mechanism of action, all checkpoint inhibitors engage the immune system and can cause unwanted immune-related reactions as a result. In the case of pembrolizumab, PD-1 interactions are crucial to maintaining immune tolerance; releasing this activity can therefore trigger various autoimmune conditions. These diseases include colitis, which resembles inflammatory bowel disease, liver toxicity, adrenal and thyroid disorders, and even type 1 diabetes in a minority of patients. Mild to life-threatening inflammation can arise in the kidneys, lungs and other organs.
Patients commonly experience skin rash, fatigue, nausea, diarrhea, decreased appetite, shortness of breath, headaches, musculoskeletal pain and more.
Combination treatments incur their own additional risks. For example, administering pembrolizumab with axitinib, a targeted cancer drug, can heighten liver toxicity.
Blood tests are required before and after each infusion to closely monitor for any potential adverse effects. Treatment may be stopped altogether if adverse reactions are too severe.
Future Directions
Pembrolizumab has evolved significantly since its release over ten years ago—not in its formula, but in its application and therapeutic potential. Ongoing research continues to refine the therapy’s efficacy and safety profile. Future research endeavors aim to answer crucial questions, such as whether to give pembrolizumab before and after surgery, how to synergize the drug with other cancer treatment modalities, and how adjustments in dosing or scheduling impact patient survival and overall response rates.
Takeaways
Pembrolizumab is a transformative anticancer drug with broad applicability and meaningful impact on patient survival. The treatment demonstrates notable response rates for several advanced cancers, many of which resist other cancer treatments. Looking ahead, research will continue to clarify pembrolizumab’s potential synergies with other treatments and its optimal use in various cancer settings. Future articles will discuss checkpoint inhibitors that employ a similar mechanism of action.
Read the full article here