Next month, the Food and Drug Administration will decide on the regulatory approval of two gene therapies for sickle cell disease. Exa-cel and lovo-cel are potentially one-time treatment options for SCD patients burdened by unmet needs.
The two treatments—exa-cel (exagamglogene autotemcel) and lovo-cel (lovotibeglogene autotemcel)—attack SCD at its genetic root. In the case of exa-cel,* a patient’s blood stem cells are extracted and then edited via CRISPR/Cas9 to express fetal hemoglobin and subsequently returned to the patient’s body. CRISPR/Cas9 is a component of the bacterial immune system that can be used to cut and edit DNA. As such, it is called a gene editing tool.
Lovo-cel works differently. It inserts a functional human beta-globin gene into a patient’s own hematopoietic stem cells and then returns the modified cells to the patient through an autologous stem cell transplantation.
On Tuesday, advisors to the FDA reviewed exa-cel to evaluate whether more research is needed into possible unintended consequences of the treatment. Specifically, the panel considered what could occur if exa-cel edits parts of the genetic code that it’s not supposed to. The committee concluded that exa-cel is sufficiently safe but suggested long-term monitoring of patients.
The other SCD gene therapy that may get FDA approval in December, lovo-cel, didn’t have a separate FDA advisory committee.
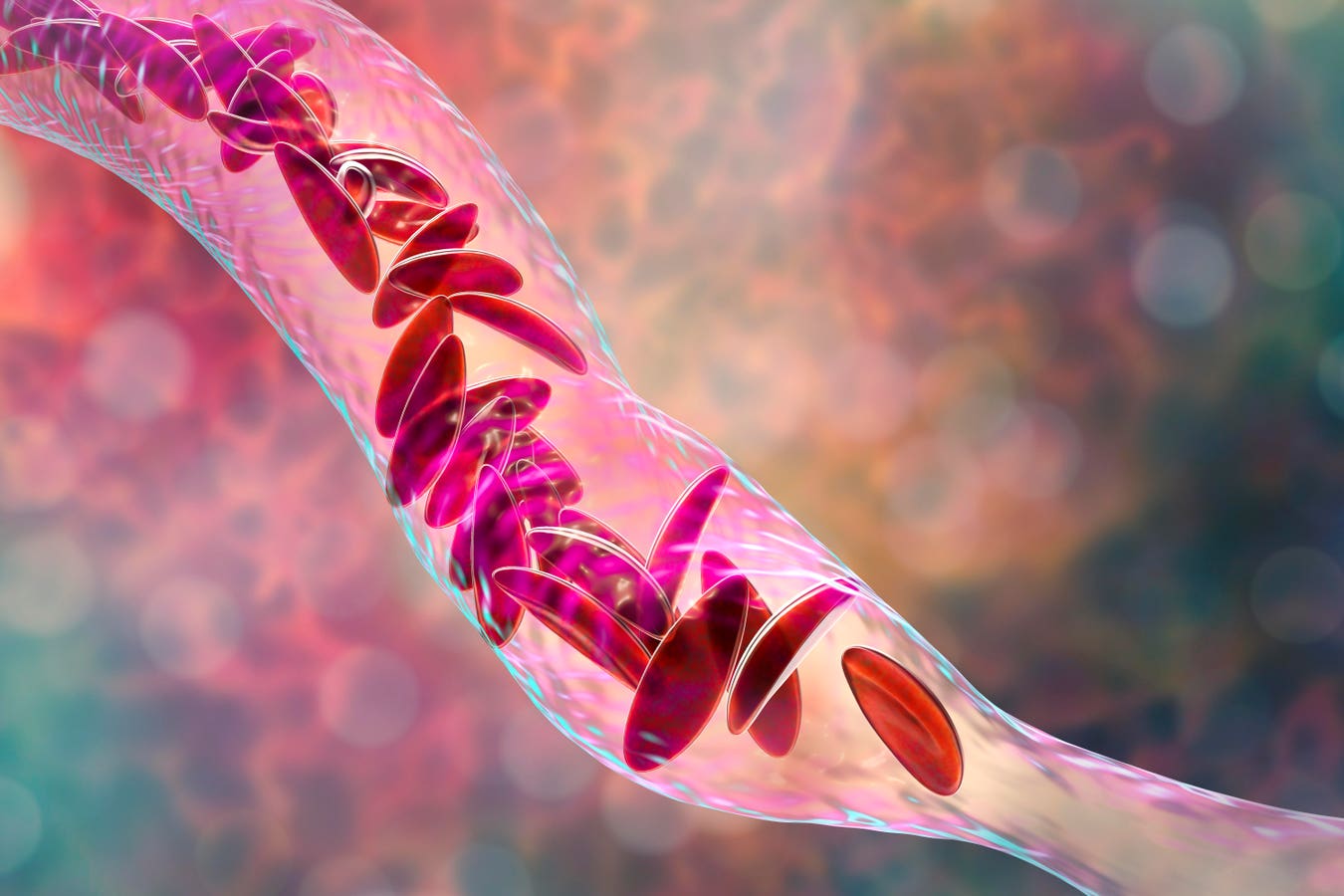
Sickle cell disease is a group of congenital red blood cell disorders, named sickle cell for the crescent shape of red blood cells. SCD alters the structure of hemoglobin, the molecule in red blood cells that delivers oxygen to organs and tissue throughout the body. The most common SCD disorder type is sickle cell anemia.
The sickle-shaped cells stick to blood vessel walls, causing blockages called vaso-occlusion that impede the proper flow of blood and therefore oxygen throughout the body. Excruciating episodes of pain often result.
The disease affects millions of people worldwide and approximately 100,000 in the U.S., most of whom are of sub-Saharan African descent.
Famous celebrities who’ve endured SCD include the jazz musician Miles Davis. The disease caused Davis chronic severe pain as well as a permanent problem with bones chipping in his wrists and hips. Except for symptomatic relief, during Davis’s lifetime there was very little available to address the disease itself.
However, this has begun to change in the past five years or so. Encouraging signs for SCD sufferers emerged a few years ago when several promising new therapies hit the market, including Adakveo (crizanlizumab) and Oxbryta (voxelotor). Adakveo is a targeted therapy that inhibits selectin, a substance which contributes to red blood cells sticking together. It can reduce number of pain crises by 50% and improve the long-term health of patients’ blood vessels. Oxbryta works to prevent red blood cells from binding together and forming the sickle shape.
But to date nothing has emerged with quite the same disease-modifying potential as exa-cel and lovo-cel. In the U.S., as many as 25,000 patients could be eligible for these gene therapies. If successful, such treatments could address some of the acute and chronic challenges facing patients. The treatments also provide the intangible, yet important benefit of hope for patients who’ve endured agony for so long.
Still, as advanced gene therapies for SCD near approval, the challenge of funding them looms large. Medicaid will be the predominant payer and it must figure out a budget-conscious way to pay for these potential one-time “cures.”
Medicaid provides healthcare coverage to tens of millions of Americans, including eligible low-income adults and children. The program is administered by states in accordance with certain federal requirements. Funding is split between states and the federal government.
For individual Medicaid state agencies the large upfront costs of gene therapies present a substantial challenge.
The Centers for Medicare and Medicaid Services is planning to pursue “testing of payment models” based on outcomes-based agreements for cell and gene therapies on behalf of all 50 state Medicaid programs. This could help Medicaid beneficiaries gain access to high-cost specialty treatments such as exa-cel and lovo-cel. However, these payment models are in an early stage of development and testing and may not be ready to implement for the new SCD therapies.
In the meantime, at the state level Medicaid programs must evaluate the feasibility of paying for exa-cel and lovo-cel, based on clinical- and cost-effectiveness assessments as well as analyses of the financial exposure they face should they decide to reimburse.
There’s some good news on this front. In April, the Institute for Clinical and Economic Review issued a draft report on the cost-effectiveness of exa-cel and lovo-cel. ICER noted that the proportion of patients achieving treatment success was estimated at 97% for both therapies. Even at the placeholder price of nearly $2 million per dose, ICER says both treatments could be cost-effective once approved by the FDA. ICER did caution that a prerequisite for these therapies’ cost-effectiveness was their durability over time and the establishment of value-based pricing agreements between payers and manufacturers.
At a price of $2 million per treatment course for lovo-cel or exa-cel, approximately 15% of all SCD patients could be infused over the span of five years without crossing a budgetary- threshold of $777 million per year. To arrive at this number, ICER estimated the growth in drug spending per year and divided that increase by the expected new molecular entrants into the market.
Too often the conversation around a therapy’s price is fixated on eye-popping dollar numbers and not value. It’s laudable that ICER redirected the public to a discussion of value. In some instances, high prices may be justified; in others, not.
It’s known that treatments with high per unit prices can nonetheless be cost-effective. Relatively expensive hepatitis C and HIV drugs, for example, but also chronic myeloid leukemia medicines, are cost-effective. In part this is due to the considerable benefits provided by these therapeutics. Additionally, these pharmaceuticals can displace other costs in the system, including hospital inpatient and outpatient clinic costs.
Cost-effectiveness and budgetary thresholds aren’t the only considerations payers, physicians and patients must take into account. According to Courtney Rice, Principal at Acadia Strategy Partners, both exa-cel and lovo-cel rely on a myeloablative busulfan conditioning regimen to prepare patients for infusion. This preparatory workup can give rise to severe adverse effects, including infertility. Accordingly, this constitutes a possible barrier to patient uptake.
In the end, once a payment system is established, individual patient preferences will be an important factor determining the adoption of exa-cel and lovo-cel should they get licensed for marketing.
Read the full article here